ЧбБЙЙйРЬПРРЧОрЧАЧљШИ ДКНКЗЙХЭ БЙЙЎ ХзНКЦЎЦФРЯ
|
 |
 |
 |
| |
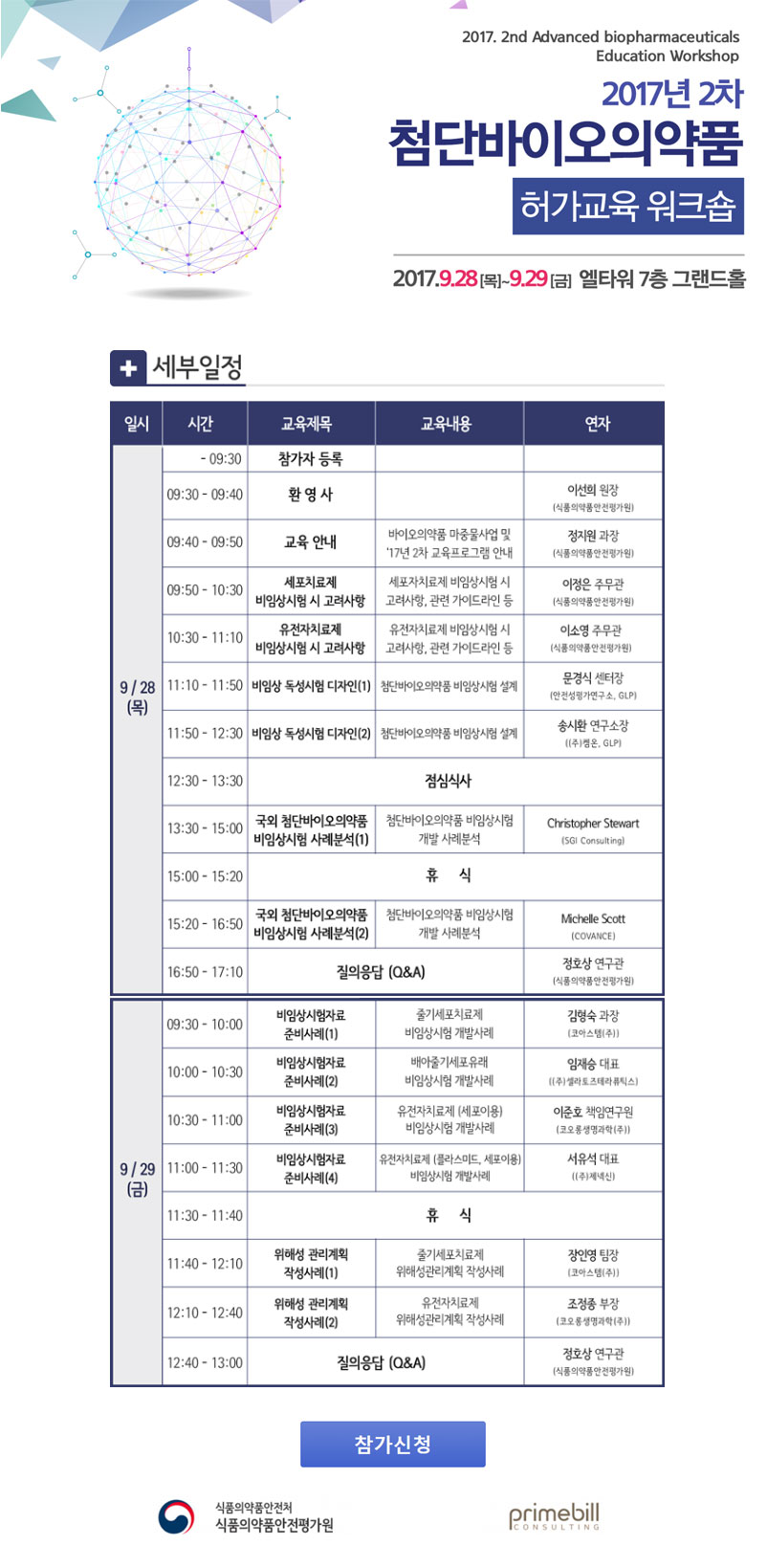
РЧАпСЖШИ
|
ЁКСЄИЦСжЛчПы СЄЛѓ ЛчЖї ИщПЊБлЗЮКвИАРЧ ОШРќМКЁЄРЏШПМК ЦђАЁ АЁРЬЕхЖѓРЮЁЛ СІСЄ(ОШ) РЧАпСЖШИ(~10/19БюСі)
<ИёРћ>
IVIGПЁ ДыЧб РгЛѓНУЧшРЧ АшШЙ Йз ЦђАЁЙцЙ§ Ею РгЛѓНУЧш НЧНУПЁ ЧЪПфЧб БЧАэЛчЧзРЛ РгЛѓНУЧш МіЧрРкПЁАд СІАјЧдАњ ЕПНУПЁ РгЛѓНУЧш АшШЙМРЧ НЩЛчПЁ ШАПыЧЯПЉ РгЛѓНУЧшРЧ ЧЅСиШ⋅БЙСІШЧдРИЗЮНс, СІЧАПЁ ДыЧб ОШРќМКАњ РЏШПМКРЛ ШЎКИЧЯДТ ЕЅ Бз ИёРћРЬ РжДй.
<СжПф ГЛПы>
1. РЯЙнРћ АэЗСЛчЧз (РЏШПМК, ОШРќМК)
2. ЧАИёЧуАЁ НХУЛ НУ АэЗСЛчЧз
3. ЧАИёЧуАЁ ШФ СІСЖЙцЙ§ КЏАц НУ АэЗСЛчЧз
<РЧАп СІУт>
БтЧб : 2017. 10. 19. (Иё)
СІУтУГ : info@kobia.kr
СІСЄОШ ШЎРЮ |
|
 |
РдЙ§ПЙАэ |
РЧОрЧА ЕюРЧ ОШРќПЁ АќЧб БдФЂ РЯКЮАГСЄЗЩ(ОШ) РдЙ§ПЙАэ ОЫИВ
<АГСЄРЬРЏ>
РЧОрЧА СІСЖОїРк ЕюРЬ СІСЖОїЧуАЁИІ ЕцЧЯПЉ ЧАИёЧуАЁ(НХАэ) ЕюРЛ ЙоРК РЧОрЧАРЛ ЕПЙАПыРИЗЮ ЛчПыЧЯБт РЇЧб ИёРћРИЗЮ ЦЧИХЧЯБт РЇЧи АќЗУ СпОгЧрСЄБтАќРЧ РхПЁАд ЧЪПфЧб ЧуАЁ НЩЛч ЕюРЛ НХУЛЧб АцПь, СІСЖОї ЧуАЁПЭ СІСЖ Йз ЧАСњАќИЎБтСи ЦђАЁ ЕюРЛ ИщСІЧЯБт РЇЧЯПЉ РЬЙЬ ЙпБоЕЧОю РжДТ СІСЖОї ЧуАЁСѕ, СІСЖ Йз ЧАСњАќИЎБтСи РћЧеЦЧСЄМ ЕюРЛ ЧиДч СпОгЧрСЄБтАќРЧ РхПЁАд СІАјЧв Мі РжДТ БйАХИІ ИЖЗУЧЯДТ ЧбЦэ,
РгЛѓНУЧш Сп ЛчИСРЛ УЪЗЁЧЯАХГЊ Л§ИэРЛ РЇЧљЧЯДТ ОрЙАРЬЛѓЙнРР ЙпЛ§ НУ КИАэБтЧбПЁ ДыЧб ШЅМБ ЙпЛ§РЛ ЙцСіЧЯБт РЇЧЯПЉ БдСЄРЛ ИэШЎЧЯАд ЧЯДТ Ею Бз ЙлПЁ ЧіЧр СІЕЕРЧ ПюПЕЛѓ ГЊХИГ РЯКЮ ЙЬКёСЁРЛ АГМБ․КИПЯЧЯАэРк Чд
<СжПфГЛПы>
1. ШЏРк ФЁЗсБтШИ ШЎДыИІ РЇЧб РгЛѓНУЧш СЄКИ ЕюЗЯСІЕЕ ЕЕРд(ОШ СІ30СЖ Йз СІ31СЖ)
| БтСИРЧ ФЁЗсСІПЁ КёЧи ШЙБтРћ ШПАњИІ АЁСј НХОр АГЙпРЛ РЇЧб РгЛѓНУЧшПЁ ТќПЉЧЯДТ АЭРК СпСѕСњШЏРЛ ОЮАэ РжДТ ШЏРкЕщАњ Бз АЁСЗЕщПЁАдДТ ИЖСіИЗ ФЁЗсБтШИАЁ ЕЩ Мі РжРНПЁЕЕ, РгЛѓНУЧшАшШЙ НТРЮПЁ АќЧб СЄКИРЧ АјАГАЁ СІЧбРћРИЗЮ РЬЗчОюСіАэ РжОю РЬПЁ ДыЧб СЂБйМКРЛ СІАэЧЯАэ РгЛѓНУЧш СЄКИ АќИЎРЧ ШПРВМК Йз СЄШЎМКРЛ ЕЕИ№ЧЯАэРк РгЛѓНУЧш СЄКИ ЕюЗЯ Йз АјАГ СІЕЕПЁ АќЧб БйАХИІ ИЖЗУЧд |
2. РЇЧи(ъЫњЊ)РЧОрЧАЕю ШИМіАсАњ АјАГ БйАХ ИЖЗУ(ОШ СІ90СЖ)
3. РЧОрЧА СІСЖ Йз ЧАСњАќИЎБтСи РћЧеЦЧСЄМ РчЙпБо Р§Тї ИЖЗУ(ОШ СІ96СЖ Ею)
РЧОрЧАНЧЛчЛѓШЃЧљЗТБтБИ(PIC/S) АЁРд(`14.7.) Йз БЙСІСЖШИІ РЇЧи РЧОрЧА СІСЖ Йз ЧАСњАќИЎ(GMP) РћЧеЦЧСЄМ ЙпБоСІЕЕАЁ ЕЕРд(`14.10.10. АГСЄ, `14.12.19. НУЧр)ЕЧОњРИГЊ, ЦЧСЄМ ЙпБо ШФ РчЙпБо, СіРЇНТАшИІ РЇЧб Й§РћБйАХАЁ ОјОю РЧОрЧАЕю СІСЖОїРкАЁ GMP РћЧеЦЧСЄМИІ ЙпБоЙоРК ШФ КаНЧ, МеЛѓЕЧАХГЊ, СІСЖОїРЧ ОчЕЕЁЄОчМі, ЛѓМг ЕюПЁ ЕћЖѓ РћЧеЦЧСЄМРЧ РчЙпБо ЖЧДТ СіРЇИІ НТАшЧЯАэРк ЧЯДТ АцПь НХБд GMP НХУЛМ СІУтПЁ ЕћИЅ КЮДуРЬ МіЙнЕЧДТЙй, РЬЗЏЧб КвЦэРЛ ЧиМвЧЯБт РЇЧи GMP РћЧеЦЧСЄМРЧ РчЙпБо Йз СіРЇНТАшИІ РЇЧб Й§Рћ БйАХИІ ИЖЗУЧд
|
4. РгЛѓНУЧш ОрЙАРЬЛѓЙнРР КИАэБтЧб ИэШЎШ(ОШ КАЧЅ 4) Ею
<РЧАп СІУт>
БтЧб : 2017. 10. 26. (Иё)
СІУтУГ : info@kobia.kr
РЯКЮАГСЄЗЩ(ОШ) ШЎРЮ |
 |
 |
| |
 |
|
| |
 |
| НХУЛРк |
НТРЮРЯ |
СІЧАИэ |
РгЛѓ |
ДмАш |
| ЛяМКЙйРЬПРПЁЧЧНК СжНФШИЛч |
20170918 |
НХЛ§ЧїАќМК ПЌЗЩ АќЗУ ШВЙнКЏМКРЬ РжДТ НУЧшДыЛѓРкПЁМ SB11(ЖѓДЯКёСжИП ЕПЕюЛ§ЙАРЧОрЧА)Ањ ЗчМОЦМНК? АЃ РЏШПМК, ОШРќМК, ОрЕПЧа Йз ИщПЊПјМКРЛ КёБГЧЯДТ СІ 3Лѓ, ЙЋРлРЇ ЙшСЄ, РЬСп ДЋАЁИВ, ЦђЧрБК, ДйБтАќ РгЛѓНУЧш |
3Лѓ |
SB11Сж 10
(
ЙаИЎБзЖї/ЙаИЎИЎХЭ)
SB11 |
| СјПјЛ§ИэАњЧа(Сж) |
20170914 |
CELLECTRA? 2000 (Electroporation, EP)ИІ ЛчПыЧЯПЉ ЧЧГЛ(Intradermal, ID)ПЁ СЂСОЧЯДТ GLS-5300РЧ ОШРќМК, ГЛОрМК Йз ИщПЊПјМКРЛ ХНЛіЧЯБт РЇЧб АјАГ, ПыЗЎ СѕЗЎ, СІ I/IIa РгЛѓНУЧш |
1Лѓ |
GLS-5300 |
| ЧбБЙОжКъКё(Сж) |
20170914 |
РћОюЕЕ 1АЁСі РЬЛѓРЧ КёЛ§ЙАЧаРћ СњКДСЖР§ЧзЗљИЖЦМНКСІ(DMARD)ПЁ КвУцКаЧб ЙнРРРЛ КИРЮ РЬЗТРЬ РжДТ ШАЕПМК АЧМБМК АќР§ПА ШЏРкИІ ДыЛѓРИЗЮ ABT-494ИІ РЇОр Йз AdalimumabАњ КёБГ ЦђАЁЧЯДТ СІ3Лѓ, ЙЋРлРЇЙшСЄ, РЬСпДЋАЁИВ РгЛѓНУЧш - SELECT - PsA 1
|
1Лѓ |
ABT494 |
| ЧбБЙШРЬРкСІОр(Сж) |
20170914 |
ДйКаР§ БтБт АэСЄМњАњ ЧдВВ МБХУРћ АГЙц УДУп ШФЙц РЏЧеМњРЛ ЙоДТ МКРЮПЁМ ШВЛіЦїЕЕЛѓБИБе 4-ЧзПј ЙщНХ(SA4Ag)РЧ ОШРќМКАњ РЏШПМКРЛ ЦђАЁЧЯБт РЇЧб СІ 2b Лѓ, ЙЋРлРЇЙшСЄ, РЬСпДЋАЁИВ, РЇОр ДыСЖ РгЛѓНУЧш |
2Лѓ |
PF-06290510 |
| ЧбБЙЗЮНД |
20170913 |
РЬРќПЁ ОрЙАРЛ ХѕПЉЙоРК РћРЬ ОјДТ СјЧрМК BRAFV600 ОпЛ§Чќ ШцЛіСО ШЏРкПЁМ ФкКёИоЦМДе + ОЦХзСЙИЎСжИП VS ЦшКъЗбИЎСжИПРЧ РЏШПМКАњ ОШРќМКРЛ ПЌБИЧЯДТ СІ 3Лѓ, АјАГ, ДйБтАќ, 2БК, ЙЋРлРЇЙшСЄ РгЛѓНУЧш |
1Лѓ |
ЦМНыЦЎИЏ(RO554-1267) |
ЧбБЙОЦНКЦЎЖѓ
СІГзФЋ |
20170913 |
ЧѲКвАЁДЩЧб АЃММЦїОЯРЧ 1Тї МБХУ ФЁЗсЗЮМ DurvalumabАњ TremelimumabРЧ ЙЋРлРЇЙшСЄ, АјАГЖѓКЇ, ДйБтАќ, СІIIIЛѓ РгЛѓНУЧш (HIMALAYA) |
3Лѓ |
MEDI4736, Tremelimumab
|
|
| |
| |
|
| |
 |
| |
|
| FDA |
|
Drug Name and
FDA Appl. # |
Active Ingredients |
Submission
Classification |
Company |
Approval Date |
DAPTOMYCIN
NDA #208385 |
DAPTOMYCIN |
НХСІЧќ ЖЧДТ НХСІСЖПј |
SAGENT PHARMS |
09/12/2017 |
ALIQOPA
NDA #209936 |
COPANLISIB |
НХЙАСњ |
BAYER HEALTHCARE PHARMS |
09/14/2017 |
MVASI
BLA #761028 |
BEVACIZUMAB-AWWB |
- |
AMGEN INC |
09/14/2017 |
ADZENYS ER
NDA #204325 |
AMPHETAMINE |
НХПыЗЎ |
NEOS THERAPS INC |
09/15/2017 |
SOLOSEC
NDA #209363 |
SECNIDAZOLE |
НХЙАСњ |
SYMBIOMIX THERAPEUTICS LLC |
09/15/2017 |
|
|
 |
| EMA |
|
| Name |
Active Substance |
Therapeutic area |
Date of authorisation/ refusal |
Mavenclad |
cladribine |
Multiple Sclerosis |
22/08/2017 |
|
|
 |
|
| |
| |
|
| |
| Clinical.gov ЙЬБЙ |
NCT
Number |
Title |
Conditions |
Interventions |
Sponsor
/Collaborators |
Phases |
| NCT03285607 |
MCS110 Combined With Neoadjuvant Doxorubicin, Cyclophosphamide, and Weekly Paclitaxel in Patients With Hormone-Receptor Positive and HER2- Breast Cancer |
Breast Cancer
Cancer of Breast |
Biological: MCS110
Drug: Doxorubicin
Drug: Cyclophosphamide
Drug: Paclitaxel
Procedure: Bone marrow aspirate
Procedure: Peripheral blood samples
Procedure: Tumor tissue |
Washington University School of Medicine|Novartis Pharmaceuticals |
Phase 1 |
| NCT03283917 |
Study of Daratumumab, Ixazomib, and Dexamethasone in Previously Treated Amyloid Light Chain (AL) Amyloidosis |
Hematopoietic
/Lymphoid Cancer
Amyloid Light Chain (AL) Amyloidosis |
Drug: Daratumumab
Drug: Ixazomib
Drug: Dexamethasone |
M.D. Anderson Cancer Center
Janssen Scientific Affairs, LC
Takeda |
Phase 1 |
| NCT03283631 |
Intracerebral EGFR-vIII CAR-T Cells for Recurrent GBM |
Recurrent Glioblastoma Multiforme
Recurrent Brain Tumor, Adult |
Biological: EGFRvIII-CARs |
Gary Archer Ph.D.
National Cancer Institute (NCI)
Duke Cancer Institute
Duke University |
Phase 1 |
| NCT03283137 |
Combination of Pembrolizumab With TGR-1202 in Patients With Relapsed/Refractory CLL and B-cell NHL |
CLL
B-cell Non Hodgkin Lymphoma |
Drug: TGR-1202
Drug: Pembrolizumab |
University of Chicago |
Phase 1 |
| NCT03283046 |
Study of Lenalidomide/Dexamethasone With Nivolumab and Ipilimumab in Patients With Newly Diagnosed Multiple Myeloma |
Malignant Neoplasms Stated as Primary Lymphoid Haematopoietic|Multiple Myeloma |
Drug: Nivolumab
Drug: Lenalidomide
Drug: Dexamethasone
Drug: Ipilimumab |
M.D. Anderson Cancer Center|Bristol-Myers Squibb |
Phase 1 |
| NCT03282344 |
A Study of NKTR-214 in Combination With Nivolumab in Patients With Metastatic and/or Locally Advanced Sarcoma |
SARCOMA |
Drug: NKTR-214
Drug: Nivolumab |
Memorial Sloan Kettering Cancer Center
M.D. Anderson Cancer Center |
Phase 2 |
| NCT03283319 |
Panblok H7 Vaccine Adjuvanted With AS03 or MF59 Antigens |
Influenza, Human |
Biological: 3.75 ug Panblok H7
Biological: 7.5 ug Panblok H7
Biological: 15 ug Panblok H7
Biological: MF59
Biological: AS03 |
Biomedical Advanced Research and Development Authority
Rho, Inc. |
Phase 2 |
| NCT03282240 |
Safety and Immunogenicity of High-Dose Quadrivalent Influenza Vaccine in Participants ЁУ65 Years in the US |
Influenza |
Biological: QIV-HD
Biological: Licensed TIV-HD1
Biological: Investigational TIV-HD2 |
Sanofi Pasteur, a Sanofi Company
Sanofi |
Phase 3 |
|
 |
| Clinical.gov РЏЗД |
NCT
Number |
Title |
Conditions |
Interventions |
Sponsor
/Collaborators |
Phases |
КёАэ |
| NCT03286751 |
A Comparative Study of LY900014 to Insulin Lispro (Humalog) in Healthy Participants |
Diabetes Mellitus, Type 2 |
Drug: LY900014
Drug: Insulin Lispro |
Eli Lilly and Company |
Phase 1 |
ЕЖРЯ |
|
|
| |
 |
| |
|
|
 |
 |
 |
 |
| |
 |
| |
|
|
 |
|
|
|
|
|
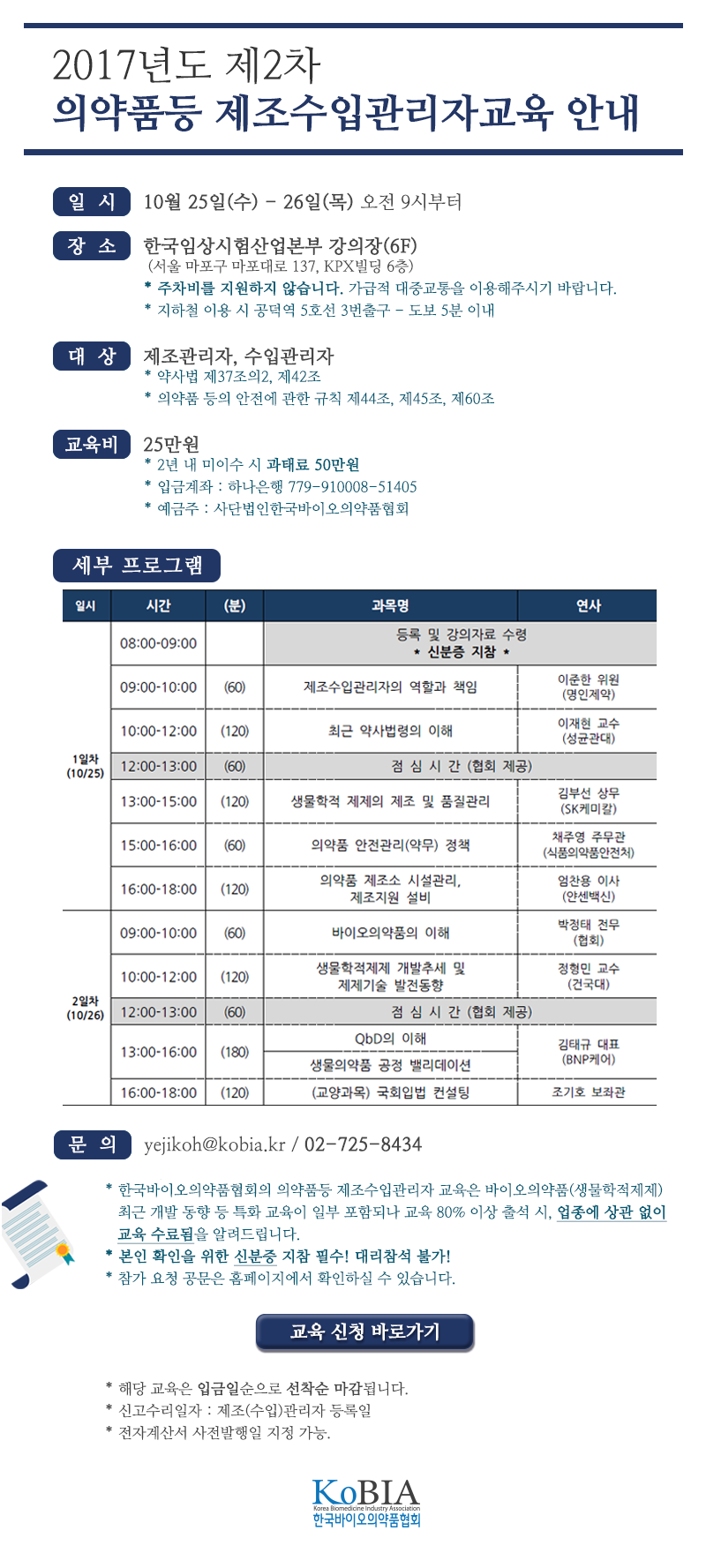
 > СЄКИИЖДч >
> СЄКИИЖДч >
 > СЄКИИЖДч >
> СЄКИИЖДч >