Biopharmaceutical Industry in Korea
 > Biopharmaceutical Industry Insight & News > Biopharmaceutical Industry in Korea
> Biopharmaceutical Industry Insight & News > Biopharmaceutical Industry in Korea
Status of Biopharmaceutical market
Sources [ www.mfds.go.kr ]
The biopharmaceutical industry is getting attention globally as the future growth engine. The importance of biopharmaceutical is the future of the pharmaceutical market and central within the context of the Fourth Industrial Revolution.
Korean government proposes to become the seventh-largest of the total global market by 2020. According to a report by GlobalData, the KoreaЁЏs Biopharmaceutical market is expected to rise to reach $20.4 billion in value by 2020. KoreaЁЏs biopharmaceutical market is worth about $2.3 billion in 2017, accounting for 12.8% share of the KoreaЁЏs pharmaceutical market worth $18.0 billion as of 2017. (Source: MFDS Yearbook 2018)
Korea is in a unique position in terms of biopharmaceutical development. The worldЁЏs first stem cell therapy was approved in Korea (ЁЏ11) and worldЁЏs first biosimilar antibody was approved in EU(ЁЏ12).
Status of Clinical Trials in Korea
Sources [ www.mfds.go.kr ІЂ www.mfds.go.kr ]
The Korean government has promoted the biopharmaceutical industry and provides significant support to the clinical trial industry. With it high quality clinical research infrastructure, well-defined regulatory and review processes, Korea has become a global clinical hub over the past decade.
Clinical trials for biopharmaceuticals are conducted quite actively in Korea. For the last 5 years, the number of clinical trial approvals for biological products has increased continuously. 213 clinical trials for biopharmaceutical products were approved in Korea in 2017.
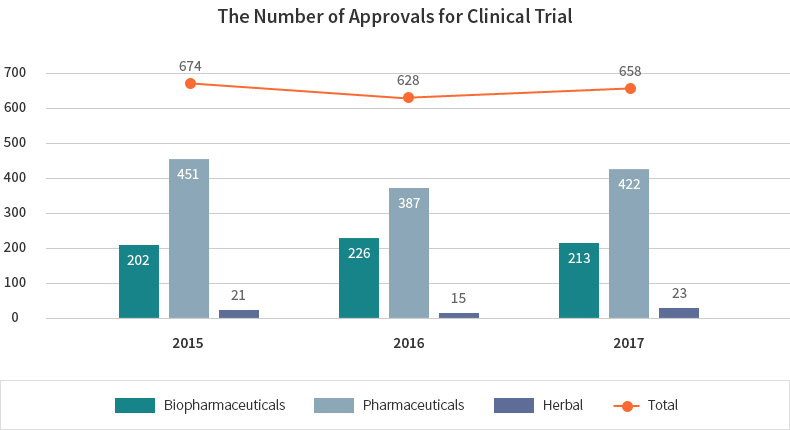
| Value | 2015 | 2016 | 2017 |
|---|---|---|---|
| Total | 674 | 628 | 658 |
| Biopharmaceuticals | 202 | 226 | 213 |
| Pharmaceuticals | 451 | 387 | 422 |
| Herbal | 21 | 15 | 23 |
The number of approvals for biopharmaceuticals is the highest for gene recombinant products(153) followed by biological products such as vaccines and blood products(31), cell therapy products(30), and gene therapy products(9) in 2017.
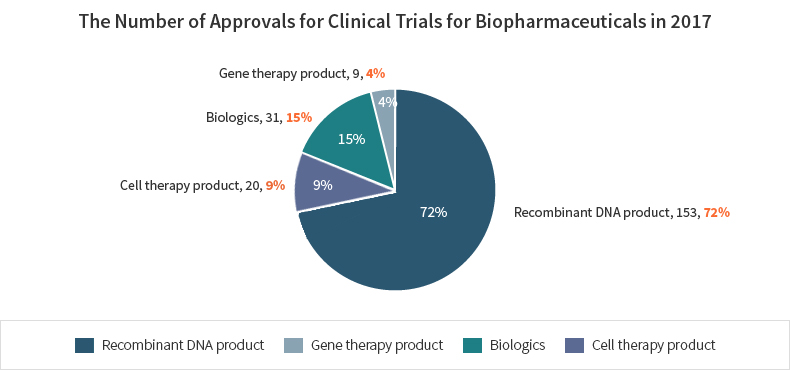
| Color | Title | Number | % |
|---|---|---|---|
| Recombinant DNA product | 153 | 72% | |
| Gene therapy product | 9 | 4% | |
| Biologics | 31 | 15% | |
| Cell therapy products | 20 | 9% |
Ёи The number of approvals for clinical trials for biopharmaceuticals
| Year | Recombinant DNA product | Cell therapy product | Biologics | Gene therapy product | Total |
|---|---|---|---|---|---|
| 2015 | 158 | 25 | 14 | 5 | 202 |
| 2016 | 151 | 33 | 33 | 9 | 226 |
| 2017 | 153 | 20 | 31 | 9 | 213 |
Source: Notice of Clinical Trial Approval Results (Mar. 2018), MFDS

